
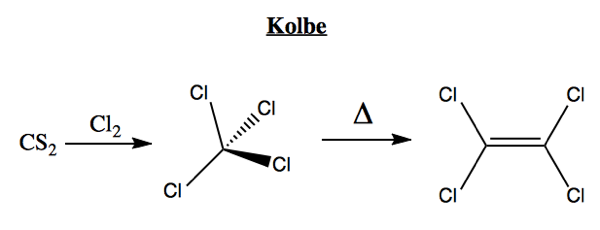
3–5 The ability to generate accurate models by extracting features directly from data without human input makes machine learning techniques an exciting avenue to explore in all areas of chemistry – from drug discovery and material design to analytical tools and synthesis planning.Įasy-to-use machine learning based tools have the potential to accelerate research and enrich education. A machine learning approach to this problem, however, might involve inputting a dataset of molecules and their respective energies into a NN, which would learn a mapping between the two. In quantum chemistry, for example, to calculate the energy of a molecule one would traditionally solve an approximation to the electronic Schrodinger equation. This makes data-driven models an interesting, and often novel, approach. 2 The natural sciences have historically relied on the development of theoretical models derived from physically-grounded fundamental equations to explain and/or predict experimental observations.

Example of chem draw software#
This resulted in much of the AI software used today, such as music/movie recommenders, speech recognition, language translation and email spam filters.ĭeep learning algorithms have been adopted by almost every academic field in the hope of solving both novel and age-old problems. The boom of big-data and increasingly powerful computational hardware allowed deep learning algorithms to achieve unprecedented accuracy on a variety of problems. A decade later, “deep learning” emerged as subclass of machine learning that employed multilayer neural networks (NNs). Although Rosenblatt proposed the perceptron in the 1950s, 1 it wasn't until the 1990s that machine learning shifted from a knowledge-based to a data-driven approach. Machine learning is a subfield of AI that focuses specifically on the “learning” aspect of the machine's intelligence, removing the need for manually coding rules. Introduction Artificial intelligence (AI) refers to the introduction of “human intelligence” into artificial machines. The ensemble model achieved an accuracy of 76% on hand-drawn hydrocarbons, increasing to 86% if the top 3 predictions were considered. By forming a committee of the trained neural networks where each network casts one vote for the predicted molecule, we achieved a nearly 10 percentage point improvement of the molecule recognition accuracy and were able to assign a confidence value for the prediction based on the number of agreeing votes. These datasets were used to train the image-to-SMILES neural network with the goal of maximizing the hand-drawn hydrocarbon recognition accuracy. Additionally, a small dataset of ∼600 hand-drawn hydrocarbon chemical structures was crowd-sourced using a phone web application. We generated a large auxiliary training dataset, based on RDKit molecular images, by combining image augmentation, image degradation and background addition. A neural image captioning approach consisting of a convolutional neural network (CNN) encoder and a long short-term memory (LSTM) decoder learned a mapping from photographs of hand-drawn hydrocarbon structures to machine-readable SMILES representations. Leveraging recent breakthroughs in machine learning, we develop ChemPix: an offline, hand-drawn hydrocarbon structure recognition tool designed to remove these barriers. Inputting molecules into chemistry software, such as quantum chemistry packages, currently requires domain expertise, expensive software and/or cumbersome procedures.


 0 kommentar(er)
0 kommentar(er)
